Biobanks are facilities that store collections of biological materials and associated information1. They are an invaluable resource for biomedical research, providing researchers with access to sample cohorts that have taken years and substantial infrastructure to collect and store. Since biobank samples are collected from patients (or healthy volunteers) using standard clinical techniques such as blood draws or biopsies, they are representative of the samples that will need to be routinely analysed when EV biomarkers are adopted into clinical practice. This makes them particularly valuable for translational research.
The aim of biobanks is usually to support various research projects across a wide range of fields on an ongoing basis. This means the exact purpose for which samples will be used is typically unknown at the time of collection. While collection and storage are standardised and well-documented, they cannot be optimised for every possible downstream application. Whenever an application area or technology emerges, researchers must ask the question – is this compatible with biobank samples? Fortunately, there is a growing body of literature to support the use of biobank samples for EV research.
Finding suitable samples for EV isolation
A huge range of different sample types exist in biobanks, with two of the most common being plasma and serum.2 The good news is that both can be used for extracellular vesicle (EV) isolation3, with size-exclusion chromatography (SEC) being one of the most widely adopted isolation methods.4 However, serum contains a substantial quantity of EVs released post-collection during blood clotting.5 Whether this is a problem, of interest, or irrelevant, depends on the research project – but it is a factor that should be considered when identifying suitable samples from a biobank. Unless platelet EVs are the population you’re researching, plasma is likely to be the most suitable sample type.
Even within plasma samples there are large variations in upstream collection and processing methods, including anticoagulant used, step timings, temperature, and centrifugation protocol.6 Each variable can affect EVs, and downstream analyses, and it is useful to understand their impact when selecting samples. As always, carefully designed and controlled experiments are key to reproducible results, and it is likely that not all biobank samples will be suitable for your EV research.
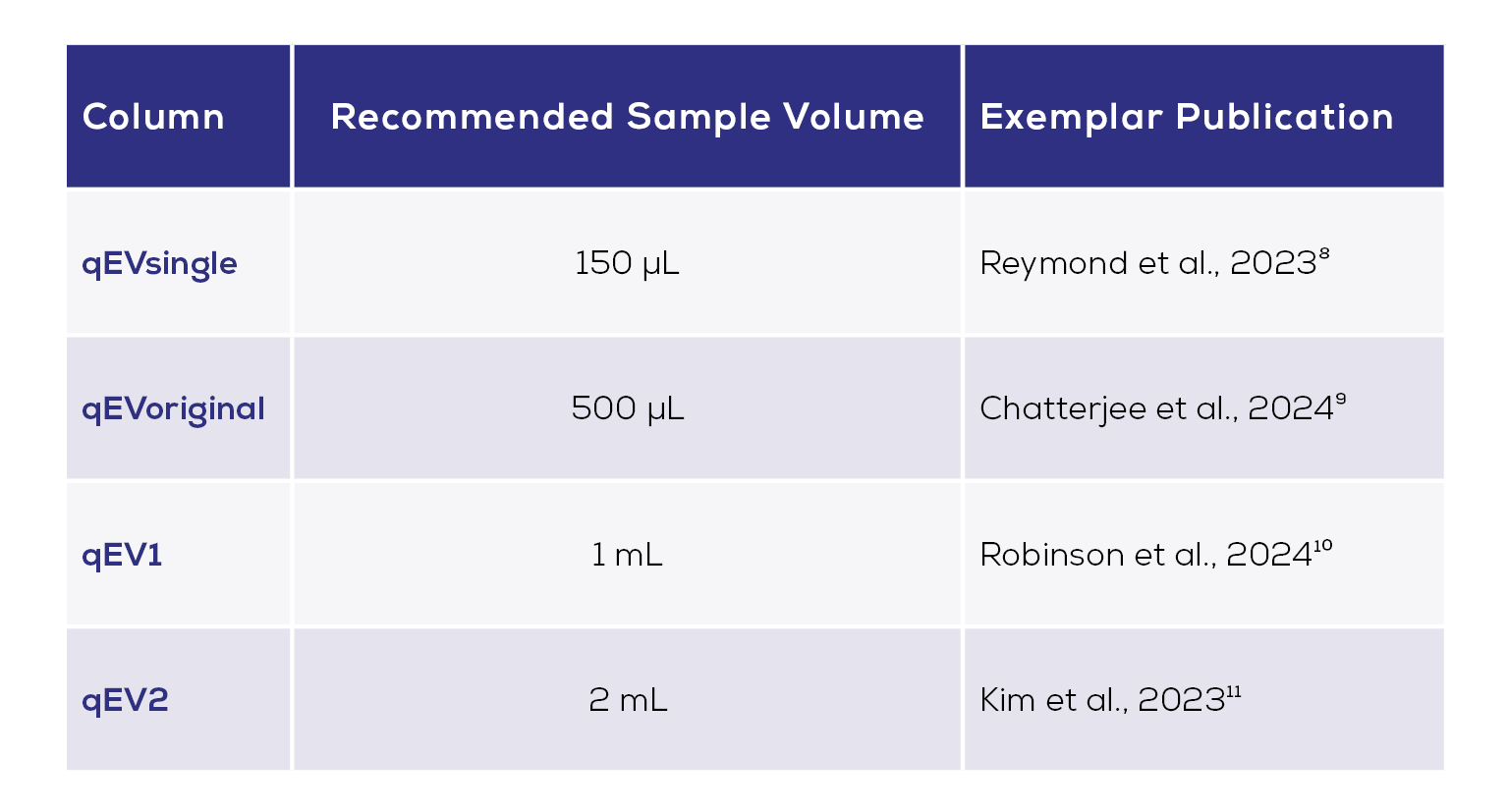
One of the potentially limiting characteristics of biobank samples is the volume available. Typical storage volumes for plasma are just 100 µL – 2 mL.7 SEC is well-suited to isolating EVs from such low volumes, with a range of qEV columns available for samples of 2 mL or less (Table 1). The ability to process these clinically relevant volumes is key for ensuring translational research methods are suitable for subsequent transition into clinical practice. Exemplar publications using small volume qEV columns to successfully isolate EVs from human plasma are given in Table 1.
Impact of storage conditions on EVs
Biobanks are long-term storage repositories, with sample collections that have often been accumulated over many years. Cohorts with long-term follow-up data or longitudinal sample collections are hugely valuable for researching chronic conditions and disease progression but require analysis of samples that have been banked for extended periods of time.
Available data on the impact of long-term plasma storage prior to EV isolation is not yet definitive. However, as with different upstream collection processes, it’s useful to understand the potential effect of storage on EV characteristics.12 A decrease in the purity of EV isolates, but no effect on EV morphology, has been reported using plasma frozen for up to 7 years prior to isolation13 and in a comparison of EVs isolated by SEC from platelet-free plasma stored at -80 °C for > 2.5 years.14 Muller et al. recommend centrifugation and ultrafiltration both before and after freezing plasma to reduce the levels of unwanted contaminants and concluded that this processing allowed recovery of immunologically-active EVs for functional studies from thawed plasma.13 Samples from long-term storage (≥ 1 year) have also been successfully used for proteomic profiling of EVs.15 It has even been suggested that storing EVs in plasma may be protective, as they are kept under more physiological conditions than when purified in buffer. 16
These examples indicate that biobanked plasma samples that have been stored for an extended timeframe can be used for EV isolation, but larger datasets are needed to fully characterise the impacts of storage on EVs and the consequences for downstream applications. It is also important to note that storage conditions have been found to have different impacts on different biofluids12 and the suitability of each should be assessed separately.
EV isolation for biobanked plasma
Since biobanks store plasma, these samples need to undergo EV purification before they can be used for EV research. There has been extensive work done in recent years on comparing and optimising different approaches to EV isolation.4,17-19 Inevitably, all methods have their strengths and weaknesses, but SEC provides a good balance of performance (high yield, purity and preservation of EV integrity) with practical considerations such as sample input requirements, cost, accessibility of required equipment and ease of processing.10, 20, 21 SEC is also scalable, with automation solutions allowing parallel processing of multiple samples (Figure 1). This not only standardises processing of cohorts for research, where minimising variability is an important part of experimental design, but will ultimately enable high-throughput clinical service delivery. Different studies will put different weights on the pluses and minuses of each isolation method, but these latter practical factors are critical for the viability of translating biomarker research into diagnostic and prognostic tests for the clinic.

A promising source of samples for EV biomedical research
Overall, biobanks are emerging as a good potential source of samples for EV research, especially if the end goal is a diagnostic application. Care must always be taken when matching samples, methods, and purpose, yet many researchers are already using biobank samples to further our understanding of EVs in health and disease.
Are you interested in SEC columns that are suitable for isolating EVs from low volumes of plasma? Or are you ready to scale up your sample processing to larger cohort sizes and need qEV automation? Please reach out to discuss how we can help.









