Drug delivery is a real challenge for therapeutics with low stability, undesirable off-target effects or other physiochemical properties which make their delivery systemically unfeasible. This necessitates a drug delivery vector of some kind, but identifying the most ideal type for different circumstances remains a challenge. Many types of nanoparticles created using many different production methods are being explored for these purposes. Among them are extracellular vesicles (EVs) which have the potential for improved targeting and high tolerability, as well as the ability to protect a range of cargo types. However, modification of EVs with specific cargo molecules in an efficient, reproducible and scalable manner is proving difficult. Towards this goal, Martinelli et al. (2020)1 have decided to go in a new direction altogether. In their 2020 study, they describe the exploration, features and production of ‘mimetic nanovesicles’ which may be more easily modified.
What are mimetic nanovesicles and how are they produced?
Mimetic nanovesicles are manufactured using a range of production techniques to encapsulate therapeutic molecules, and potentially offer a far greater production yield (reportedly by 100-fold).2 They have been described in the literature as mimetic nanoparticles, exosome mimetics, and ‘bioinspired exosome-mimetic nanovesicles’.2-5 We’ll gloss over the exosome moniker for now, and gently point out that it would be more of an EV mimetic as they aren’t produced from the endosome. Essentially, they are particles which are inspired by EVs but aren’t naturally produced. So how have they been produced by different researchers?
Jang et al. (2013) used monocytes or macrophages which were extruded through serial filters with diminishing pore sizes to create nanovesicles which carried chemotherapeutics.2 Likely these particles contained a mixture of cellular components, such as nuclear and organelle fragments, making them most similar to apoptotic bodies in composition. By breaking down and assembling different subcellular components in vitro, high-yield nanovesicles may be produced with what some researchers believe are similar biophysical and biochemical features to their naturally released EV counterparts. Whilst the jury is definitely still out on this claim, Martinelli et al. (2020) have gone some way to improving the characterisation of mimetic nanovesicles generated using cell plasma membranes.
A new method for producing drug-loaded mimetic nanovesicles
Martinelli et al. (2020) produced mimetic nanovesicles by lysing human malignant glioma T98G cells. The process then involved centrifugation to pellet the membrane debris, followed by washing and resuspension of the membrane pellets in cold PBS. The fragment membranes were then passed through 0.45 µm pore size filters and incubated at 37°C for one hour, resulting in the spontaneous generation of mimetic nanovesicles. EVs released from the same cells were also isolated and their physicochemical parameters were compared with that of the mimetic nanovesicles.
The researchers also mixed drugs (an autofluorescent plant alkaloid called Berberine, chemotherapeutic Temozolomide, and a compound with antineoplastic, anti-inflammatory and anti-angiogenic effects called Givinostat) with the isolated lysed membranes and incubated them at 37°C to generate drug-loaded mimetic nanovesicles. These drug-loaded nanovesicles were then applied to cells to investigate the cytotoxic effect of the drugs.
Physical and functional characterisation of mimetic nanovesicles
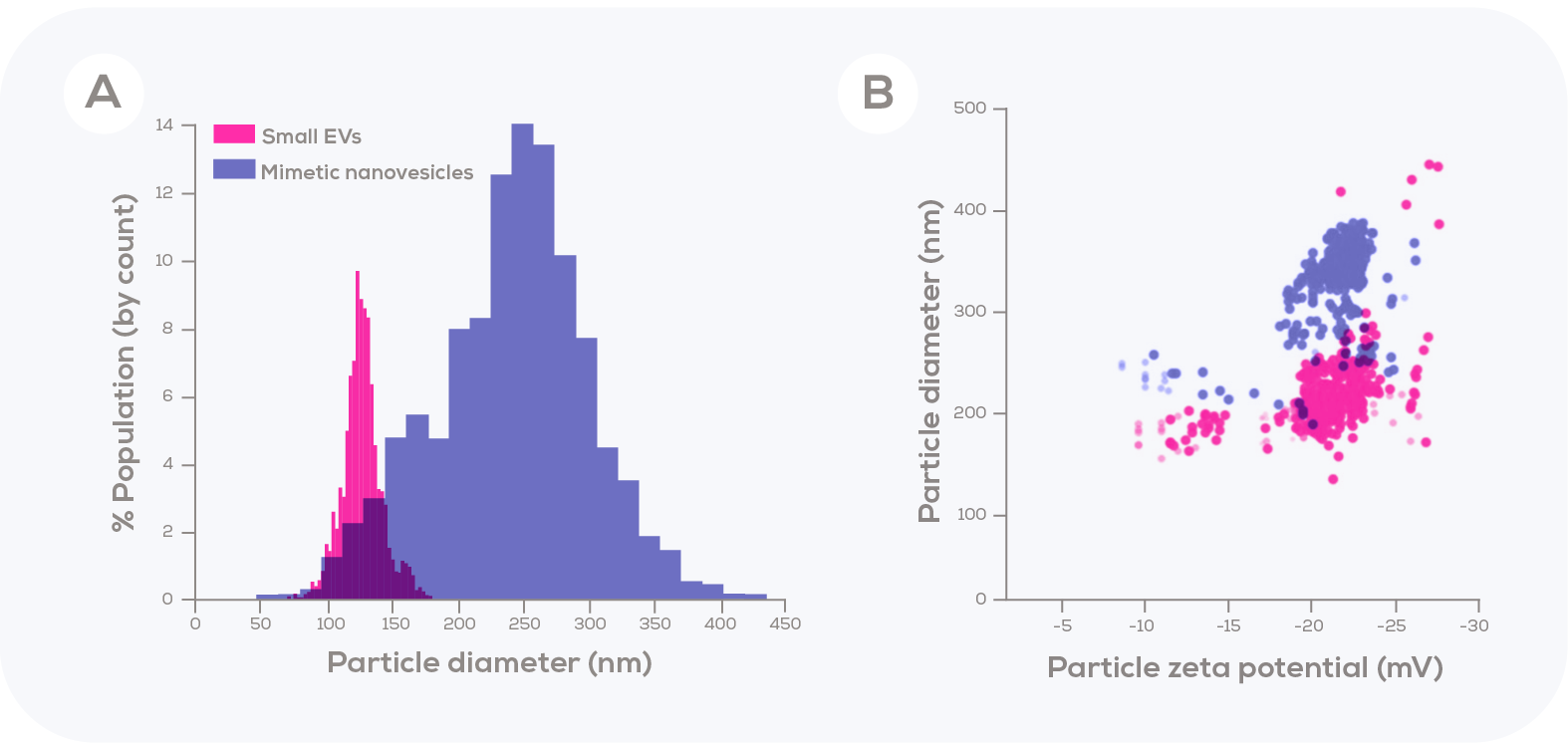
In addition to visualisation via transmission electron microscopy, Tunable Resistive Pulse Sensing (TRPS) was used to compare the concentration and particle size distribution of mimetic nanovesicles to that of the EVs generated from the same number of cells. The authors chose to compare the size of these mimetic nanovesicles to those of just the small EVs (<180 nm) secreted by the donor cells. In doing so, they found that these small EVs were clearly smaller than the mimetic nanovesicles. As such, the mimetic nanovesicles are not in the size range of exosomes, which was the author’s aim.
Fluorescent microscopy and flow cytometry assays indicated that mimetic nanovesicles were internalised by cultured T98G cells. Mimetic nanovesicles electroporated with eukaryotic vector-expressing fluorescent protein were taken up by approximately 95% of T98G cells. Importantly, exposure of T98G cultured cells to mimetic nanovesicles loaded with Temozolomide or Givinostat produced similar cytotoxic effects (i.e., ~40% decrease in cell viability) to what was observed when the free drug (at an equivalent concentration) was added directly to the cell culture. This suggests that there was no additional impact of encapsulating the drugs in the mimetic nanovesicles in vitro.
Mimetic nanovesicles: a new platform for therapeutic delivery?
Martinelli et al. (2020) showed that detergent-based cell lysis produced fragmented cell membranes and other fragments which could then be easily reassembled into mimetic nanovesicles. Furthermore, the reassembly of mimetic nanovesicles could be coupled with the presence of diverse range of molecules, generating encapsulation of those molecules into the aqueous space of the mimetic nanovesicle. Utilising the high-resolution data captured using TRPS, the authors identified that mimetic nanovesicles were larger in diameter than the isolated small EVs, which could indicate a larger capacity for drug encapsulation. While the impact of this larger size on cellular uptake and tolerability is unknown, the authors did observe the uptake of these particles in growing T989G cells followed by an induction of apoptotic cell death (as compared to non-loaded mimetic nanovesicles). This approach for generating cargo-loaded mimetic nanovesicles presents an interesting new angle for drug delivery, with the potential for many different drug combinations and targets to be explored.









